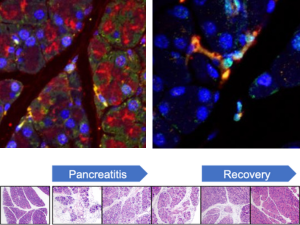
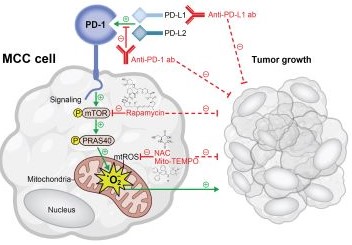
Exploring How to Enhance Drug Delivery and Efficacy Through Nanoparticles and Macroscale Materials
Natalie Artzi, PhD, a principal investigator in the Brigham and Women’s Hospital Department of Medicine, has changed our basic understanding of biomaterials under different environmental and pathological states. Her lab is dedicated to designing smart biomaterial platforms and medical devices to improve human health.
Read More...