From novel laboratory research that enables scientists to define pancreatic cancer cells better to innovative clinical approaches that help patients recover from surgery more quickly, Dana-Farber Brigham Cancer Center (DFBCC) is steadily advancing the diagnosis and management of pancreatic cancers.
Our experts are continuing to develop novel strategies for improving outcomes in pancreatic diseases, including benign conditions and pancreatic cancer,” says Chandrajit P. Raut, MD, MS, Director of the Dana-Farber Brigham Cancer Center Sarcoma and Bone Oncology Programs. “Investigators from a number of disciplines across our institution are making great strides at improving outcomes, which will directly improve the care of the patients we see.”
Reversible Fixation Overcomes Challenges of Single-Cell Profiling
Sahar Nissim, MD, PhD, of the Brigham’s Division of Genetics and Division of Gastroenterology, Hepatology and Endoscopy is an award-winning physician-scientist. His team studies the earliest events that cause a normal pancreas cell to progress toward cancer to devise “interception,” a strategy to block those events and prevent cancer from forming altogether.
Researchers in Dr. Nissim’s laboratory leverage developmental biology, genetic discovery in hereditary pancreatic cancer families, integrative transcriptomic and epigenomic approaches, and pre-clinical experiments in mouse models and human tissue to study determinants of pancreas cell identity and cancer initiation.
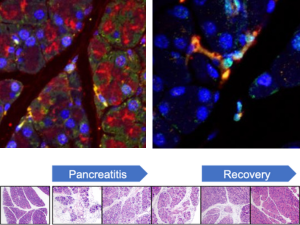
In a recent study, Dr. Nissim and first co-authors Woo-Jeong Jeong and Katherine Jean Aney presented a single-cell method to define the transcriptomes of pancreas cells, providing a vital new tool for future investigation of the pancreas in health and disease. The study, A Novel Approach for Pancreas Transcriptomics Reveals the Cellular Landscape in Homeostasis and Acute Pancreatitis, was published in Gastroenterology. It marked the first-time reversible fixation was applied to single-cell analysis of the pancreas.
Single-cell RNA-sequencing studies have advanced the understanding of signaling pathways and cell diversity in many organs. However, digestive enzymes produced in acinar cells hamper such investigation of the pancreas. FixNCut, the novel method described by Dr. Nissim and his co-authors, uses reversible fixation to achieve unprecedented single-cell transcriptomic definition of challenging pancreas cells, including acinar and immune populations in homeostasis and following acute pancreatitis.
This method overcomes the endogenous digestive enzymes that have historically challenged single-cell profiling of the pancreas, Dr. Nissim explains.
“We know that certain stressors can cause pancreatic cells to evolve and become precursor lesions to pancreatic cancer,” he says. “Studying these cells and cancer precursors will give us critical insights into why cancer forms and how we might intercept that process. However, the pancreas environment is hostile to traditional single-cell assays. Moreover, a permanent fixative approach to address this challenge precludes the ability to conduct subsequent research that requires generation and understanding of the genes expressed.”
FixNCut is a single-cell RNA sequencing approach where tissue is reversibly fixed with dithiobis (succinimidyl propionate) (DSP) prior to dissociation and single-cell preparation. The approach completely shuts off RNase, DNase, and protease activity—the activity of the digestive enzymes that traditionally impede this type of work—and then allows that fixation to be reversed for subsequent analyses.
“DSP is a cross-linker that has been considered for use as a reversible fixative,” Dr. Nissim says. “However, this is the first time anyone has harnessed this reagent for single-cell analysis of pancreas cells. This method is a transformative advance in our ability to understand the pancreas at homeostasis and disease states. These insights, in turn, will inform new strategies for prevention, early detection, and treatment.”
Aney adds that while this initial FixNCut study was conducted in mouse models, a future goal is to apply the approach to human pancreatic tissue.
“Due in part to issues around access to human pancreatic tissue, the causes of pancreatic cancer—cell types and states as well as risk factors—are more easily studied in mouse models than in humans,” she says. “FixNCut gives us the ability to better understand cell types and how they evolve so that we might intercept pancreatic cancer in different contexts.”
“This approach,” Jeong adds, “may also be applied in other organs that pose challenges for single-cell RNA sequencing and other omics pipelines as well.”
Synergistic Effects of Robotic Surgery and ERAS Pathway Lead to Early Hospital Discharge
DFBCC’s leadership in clinical innovations for pancreatic cancer was also on display in a recent retrospective study led by Thomas E. Clancy, MD, FACS, Co-Director of the Pancreatic and Biliary Tumor Center at Dana-Farber Brigham Cancer Center. The investigators aimed to assess the safety of an enhanced recovery after surgery (ERAS) and early discharge pathway in a robotic pancreatoduodenectomy (PD) (“Whipple”) program and to identify the synergistic effects of robotic surgery and an ERAS pathway on length of stay (LOS).
The study, published in the Journal of the American College of Surgeons, is the first to demonstrate the feasibility and safety of discharging patients after robotic PD on postoperative day four.
“While PD has allowed pancreatic surgeons to improve patient outcomes, it remains a challenging procedure associated with postoperative complications that can increase hospital LOS,” Dr. Clancy says. “Minimally invasive approaches, including laparoscopic surgery and robotic PD, are starting to show improved postoperative recovery and outcomes. These approaches can shrink LOS from an average of two weeks to just four days.”
The study investigated the effects of an ERAS and early discharge pathway that included perioperative goal-directed IV fluid administration, omission of nasogastric tube use, and early enteral feeding postoperatively. Patients were deemed ready for discharge if they could tolerate a diet without nausea and vomiting, had satisfactory pain control, and could ambulate independently.
Compared with patients receiving open PD, those in the robotic PD group had lower estimated blood loss and a significantly shorter postoperative hospital LOS than those receiving open PD—with 40% achieving a postoperative LOS of four days or fewer. According to the study, the early discharge appears to be safe; the robotic PD group demonstrated similar complication as well as readmission rate with no mortality.
“We were impressed with how quickly patients got better,” Dr. Clancy says. “Many were feeling almost back to normal a week or two after surgery. With a traditional open Whipple procedure, that milestone doesn’t typically occur for up to two months.”
According to Dr. Clancy, the study has important implications for optimizing resources and hospital capacity and for expanding indications for robotic PD in an era where neoadjuvant chemotherapy is used increasingly for pancreatic cancer. It also opens up new surgical options to patients who may not otherwise be offered a minimally invasive approach.
In addition, as surgeons become more experienced with robotic Whipple surgery, they can transfer the skill set to other surgical procedures, such as gastrectomy, biliary reconstructions, and bile duct tumor resection.
“We have a very experienced and diversified robotic surgery program,” Dr. Clancy says. “Thanks to philanthropic partnerships, a shared vision among leadership, and an essential support structure reflecting excellence across the board, our program continues to grow and offer advanced procedures to more patients every year.”