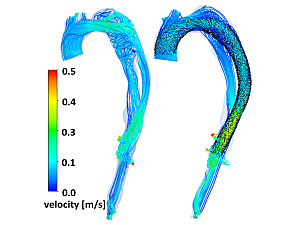
Computational fluid dynamics offer reliable means to study hemodynamic patterns in aortic dissection patients (left). Virtual surgery through numerical simulation of thoracic endovascular aortic repair implantation allows for therapy planning and device optimization (right). Image courtesy of Farhad R. Nezami, PhD.
Farhad R. Nezami, PhD, lead investigator in the Brigham and Women’s Hospital Department of Surgery, Division of Cardiac Surgery, describes himself as an “alien.” The reason? He is a mechanical and aerospace engineer by training who works closely with surgeons and physicians in the division to tackle challenges in cardiac and thoracic surgery.
To say Dr. Nezami’s research interests are diverse only hints at the remarkable range of work taking place in his lab. He and his team pull from engineering, computer science, and bioinformatics to uncover better diagnostic tools and predictors of clinical events and enhance therapeutic planning and clinical decisions.
“What I am doing has a direct translation for the way that surgery, intervention, and device development are happening,” Dr. Nezami says. “I was surprised to see that this work traditionally doesn’t happen much in the hospital setting. But this is exactly where it should take place, as opposed to mechanical engineering departments in universities, where we have no clue what’s going on in clinical practice.”
Extracting More Information From Medical Images
Imaging modalities such as CT, MRI, and echocardiogram produce abundant data. However, as Dr. Nezami says, doctors only leverage a fraction of this data to make crucial clinical decisions.
Aortic surgery provides one example. Typically, a radiologist conducts a CT scan of the aorta and then measures the diameter at a specific location. A projection of how much the aorta will grow over the ensuing year informs the decision on whether to proceed with surgery.
The driving force for this decision, Dr. Nezami notes, should not be the anatomy but rather the pathology and mechanical state that can lead to potentially catastrophic events. That means extracting more information from the images than the naked eye can easily detect.
For instance, the pattern that calcium is distributed on coronary or noncoronary cusps of a stenosed aortic valve has a significant influence on its hemodynamics. Depending on the nature of the turbulence, the patient could be at heightened risk for cardiomyopathy, aortic dissection, or aneurysm.
“I’m trying to get these images and then run simulations,” Dr. Nezami says. “We model the disturbances in the hemodynamic patterns, quantify these observations, and then provide supplementary information to the surgeons or medical doctors for the sake of severity diagnostics, risk stratification, and surgery planning.”
Conducting Virtual Surgery for Medical Devices
Dr. Nezami’s lab also conducts virtual surgeries to assess the safety and efficacy of prosthetic valves and stents, thoracic endovascular repair devices, and other medical devices.
Using numerical simulation allows Dr. Nezami and his colleagues to “implant” a device in a 3D model that depicts an individual patient’s anatomy. By observing the interaction between the device and the patient, the team can determine whether the surgical approach—as well as the size and shape of the device—are appropriate for the patient.
This process also offers insights into the likely outcome and what alternative surgical approaches may be worth considering. Dr. Nezami can share this information, along with the risks associated with each surgical alternative, so that the care team can choose the most suitable solution for the patient.
“This is another step on the path toward personalized medicine,” Dr. Nezami says. “When it comes to devices and surgery, there is no ‘one size fits all.’ Everyone’s anatomy is unique. So, when planning for an intervention or surgery, we should be looking to fine-tune the approach to that individual.”
Improving Efficacy of Life Support Devices
Heart pumps, oxygenators, and other extracorporeal life support devices are traditionally used only as a temporary bridge from critical care to therapy or transplant. Due to lengthening transplant queues, many of these tools would ideally become destination devices. But getting there would mean overcoming some major hurdles.
For example, life support devices can cause hemodynamic changes that lead to clot formation. Using flow simulation, which can gauge thrombosis risk via various biological and biochemical assays, Dr. Nezami’s lab can predict flow stasis (i.e., low stresses) or blood damage.
The lab can also run simulations to guide decisions around ideal flow rate or settings on the life support machine. Currently, critical care teams do not benefit from a feedback loop that helps them make these decisions; they must instead rely completely on their own experience.
“What flow rate works the best in terms of oxygenation for different types of patients? We don’t really know, but these simulations give us better mechanical insights,” Dr. Nezami says. “You don’t want quality of care to depend solely on the expertise of the critical care team, since that differs from institution to institution. The engineering tools we’re using can help ensure equitable treatment for all patients, no matter which hospital they go to.”
From Ideation to Application
Dr. Nezami previously spent six years at MIT, an incredible place to perform mechanical or bioengineering research. At the Brigham, however, he finds it considerably easier to learn about the challenges surgeons and physicians are facing—and then to collaborate with these same experts on devising solutions.
“Here, I’m developing a much deeper appreciation for the significance of the simulations we’re doing and understanding of how they are being applied in clinical practice,” Dr. Nezami says.