A groundbreaking intranasal vaccine for Alzheimer’s disease (AD) developed at Brigham and Women’s Hospital is being tested in its first phase of human trials. Howard L. Weiner, MD, co-director of the Ann Romney Center for Neurologic Diseases, is leading the study, which was launched in December 2021 after the FDA approved the treatment of human AD patients based on strong data in animal models.
The intranasal vaccine relies on the immune modulator Protollin, a nasal proteosome-based adjuvant used safely in humans as an adjuvant for other vaccines. The AD trial is testing Protollin’s ability to trigger monocytes in the cervical lymph nodes to migrate to the brain and clear beta-amyloid plaques—one of the hallmarks of AD—without creating or infusing beta-amyloid antibodies and inducing encephalitis.
First Phase of Alzheimer’s Trial in Progress
Ranging in age from 60 to 85 years, the 16 participants in the clinical trial all have early, symptomatic AD. They each have had a recent amyloid-positive PET scan but are in good general health with no disease expected to interfere with the study.
In the first phase, which is expected to run through the end of 2022, each participant receives two single ascending doses of the immunotherapy one week apart to measure its safety, any presence of side effects, and its efficacy in stimulating the immune system. The initial phase will end once Dr. Weiner and his team determine the correct dosage and see sufficient blood biomarker evidence of activated mononuclear cells and enhanced beta-amyloid phagocytosis, with no obvious negative effect.
“We’re almost done with this first phase, and it does appear safe,” Dr. Weiner says. “It’s taken nearly 20 years of work in the laboratory to get to this first stage, so it’s a major milestone that we’ve gotten it into Alzheimer’s patients.”
A Novel Approach to Clearing Out Amyloid
Dr. Weiner has a long history of harnessing the immune system, particularly the mucosal immune system, to treat autoimmune, neurologic, and other diseases, including multiple sclerosis. His recently published book, The Brain Under Siege, explores the cutting-edge science behind treatments for AD and other brain diseases, including a chapter in which he describes his work with Protollin.
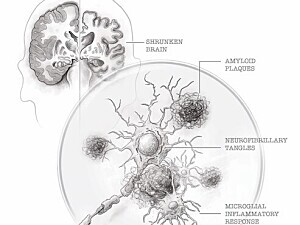
“The ‘crime scene’ in Alzheimer’s consists of three components—amyloid in the brain that triggers the tau protein, which in turn triggers activation of microglial cells,” Dr. Weiner says. “One of our big challenges with Alzheimer’s is to clear out the amyloid, and to have the body’s own immune system do that is a very novel approach.”
Tanuja Chitnis, MD, professor of Neurology at the Brigham, is principal investigator of the trial. “For 20 years, there has been growing evidence that the immune system plays a key role in eliminating beta-amyloid,” she says. “This vaccine harnesses a novel arm of the immune system to treat AD and paves the way for us to pursue a whole new avenue for potentially treating not only AD, but also other neurodegenerative diseases.”
Once they reach their goal for the first phase, Dr. Weiner, Dr. Chitnis, and their team will do a longer study with a larger cohort to examine the efficacy of more than two doses on amyloid in the brain. “The next step would be to treat a larger number of patients for a longer period of time and to look at helping cognition,” Dr. Weiner notes.
A Possible Preventative Treatment for Alzheimer’s Disease
Projected for early 2024, the trial’s third phase would enroll hundreds of patients with the ultimate goal of gaining FDA approval for the vaccine within five years. “Developing and testing these drugs always takes longer than expected, but the fact that we’re now treating people with Alzheimer’s is a major advance,” Dr. Weiner says.
In addition to treating AD patients, Dr. Weiner hopes the intranasal vaccine therapy could have applications as a preventative therapy akin to blood pressure treatments.
“If this really works, you could give the vaccine to people in their 50s or 60s who don’t have Alzheimer’s but may be symptomatic or at risk,” he says. “In that sense, it would be like treating high blood pressure to prevent strokes and heart attacks.”
Until then, Dr. Weiner emphasizes the vaccine’s simple mode of delivery and the study’s early findings around safety and efficacy. “The fact that it’s something easy to give like a nasal spray and that it doesn’t appear to have side effects would make it a very exciting and transformative discovery,” he concludes.