The chemotherapy drug docetaxel has been shown to prolong survival in metastatic prostate cancer. But can it boost the cure rate in men with high-grade, nonmetastatic prostate cancer and low levels of prostate-specific antigen (PSA), patients who traditionally have had a poor prognosis? A meta-analysis of five prospective randomized clinical trials (RCTs) found that adding docetaxel to standard-of-care treatment (SOC) in these patients was associated with a significant reduction in death from prostate cancer.
The study was published in JAMA Network Open on November 1.
‘A Marked Improvement in Survival’
Investigators performed a meta-analysis of RCTs evaluating SOC treatment with radiotherapy and androgen deprivation therapy or with radical prostatectomy versus SOC plus docetaxel. The final study cohort of 2,184 patients included 145 eligible patients (6.6%) across four eligible RCTs.
“Of these 145 patients, 139 had excellent performance status [PS] and were the main focus of the study. An excellent PS identifies patients who can tolerate the full course of chemotherapy and therefore benefit if the treatment proves effective,” says Anthony Victor D’Amico, MD, PhD, chief of Genitourinary Radiation Oncology at Brigham and Women’s Hospital and senior author of the study.
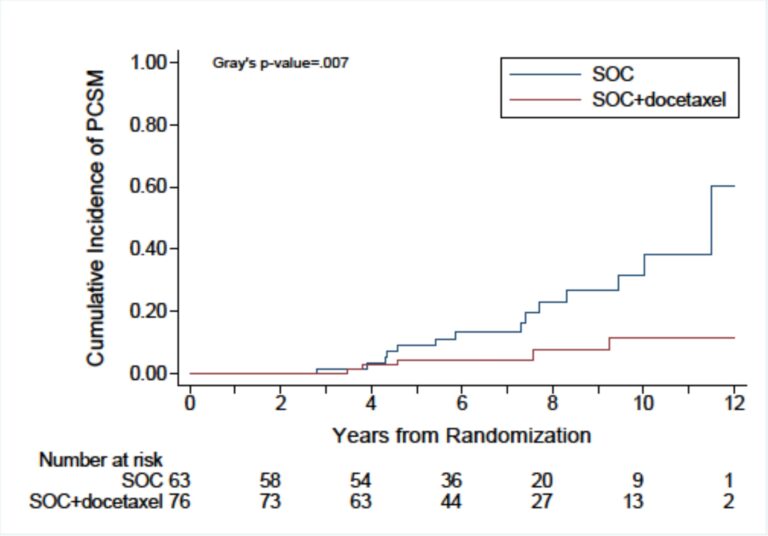
Among these 139 patients, SOC plus docetaxel was associated with a significant 70% reduction in prostate cancer-specific mortality (PCSM) and a nearly halved all-cause mortality. Remarkably, this treatment regiment dropped the 10-year PCSM rate from nearly 40% to less than 10%, leading to a 10-year overall survival that increased to 80% as compared to only 60% with SOC therapy.
“It’s a marked improvement in survival for these patients, who currently do not have any highly effective treatments,” Dr. D’Amico says.
Uncovering a Split in Survival Curves That Favored Docetaxel
Dr. D’Amico was the senior author of one of the seven RCTs studying docetaxel in men with nonmetastatic prostate cancer that were initially considered for the meta-analysis. Results from all seven studies showed no definable benefit for docetaxel.
However, in analyzing data from his team’s original trial, Dr. D’Amico and his colleagues identified a split in survival curves between four and eight years after randomization favoring docetaxel. They noted a similar finding in two of the other randomized studies that contained a large proportion of patients with high-grade prostate cancer. Subsequently, the investigators of those trials were approached about doing a meta-analysis of all the data, concentrating on the population of patients with high-grade cancer and low PSA levels who might benefit from docetaxel.
The meta-analysis included individual data for patients with nonmetastatic prostate cancer, a Gleason score of 8 to 10, and a PSA level of less than 4 ng/mL.
“It may not be intuitive that you have prostate cancer with a low or normal PSA that’s so aggressive,” Dr. D’Amico notes. “But we’ve learned that the biology of these cancers is unique in that they appear to be resistant to current SOC therapies. Specifically, these cancers have a very short response to hormonal therapy, which is a mainstay of prostate cancer treatment after radiation therapy or surgery. So, we hypothesized that this group might respond to docetaxel, which works through a mechanism of action that is different than that of conventional hormonal therapies.”
A Call to Evaluate More Treatments in These Patients
According to Dr. D’Amico, the primary limitation of the meta-analysis is the relatively small sample size, given that this cancer afflicts about 7% of all patients who present with a new diagnosis of localized or locally advanced prostate cancer. However, given the magnitude of the survival differences observed, it is likely that with time and more events, a statistically significant overall survival benefit will emerge.
“The finding with docetaxel is an important step forward for this unique group of patients, who need better treatments than we have today,” Dr. D’Amico says. “Going forward, I’d like to see more treatments evaluated in these patients, such as abiraterone and other more novel therapies.”