In a research project spanning over a decade, Brigham and Women’s Hospital scientists developed a method for simultaneously detecting thousands of lipid molecules that are displayed to T cells in the human immune system. The breakthrough has resulted in the first integrated lipidomic map for CD1 antigen-presenting molecules, which can guide the investigation of lipid T cell antigens and blockers in any cellular system or disease.
The study took place in the laboratory of D. Branch Moody, MD, principal investigator and associate physician in the Brigham’s Division of Rheumatology, Immunity and Inflammation. It was led by the Moody Lab’s Tan-Yun Cheng, PhD, senior staff scientist, and Shouxiong Huang, PhD, now at the University of Cincinnati College of Medicine, and involved a group of researchers from the University of Oxford, the United Kingdom, and Melbourne, Australia.
In a paper published in Cell in September 2023, Drs. Cheng and Huang along with their co-authors wrote, “The CD1 system binds lipid antigens for display to T cells. Here, we solved lipidomes for the four human CD1 antigen-presenting molecules, providing a map of self-lipid display. Answering a basic question, detection of more than 2000 CD1-lipid complexes demonstrates broad presentation of self-sphingolipids and phospholipids. ”
The identification of hundreds of ligands for CD1 supports the idea that the immune system can recognize and respond to many types of cellular lipids. Based on the large number of lipids studied in CD1 binding clefts (Figure 1), the data support emerging motifs or rules regarding the size and structure of lipids that can be displayed to T cells. Whereas lipid antigens rest largely inside CD1 to allow the approach of T cell receptors and activation of T cells, some large lipid blockers protrude away from the CD1 surface to block T cell activation (Figure 2). Because CD1 proteins have the same structure in nearly all humans, these lipid-binding patterns provide the basis for designing optimized ligands to activate or block T cell responses in any human patient.
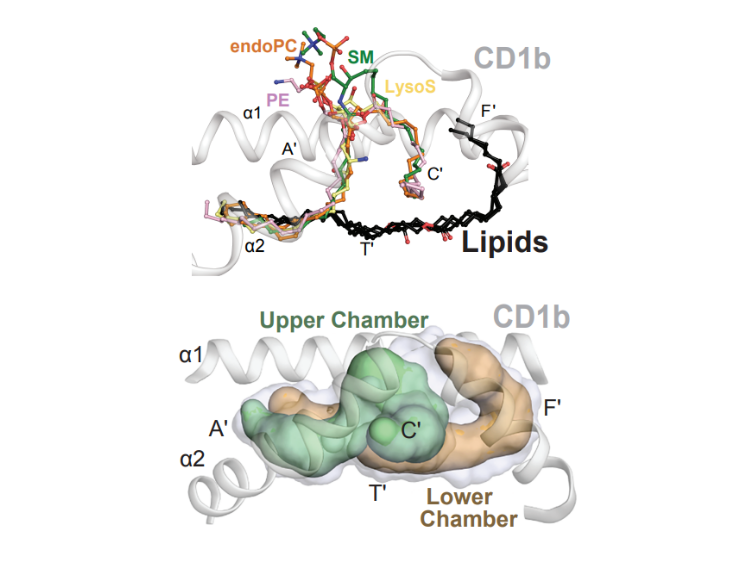
Figure 1: Antigens rest inside CD1 proteins.
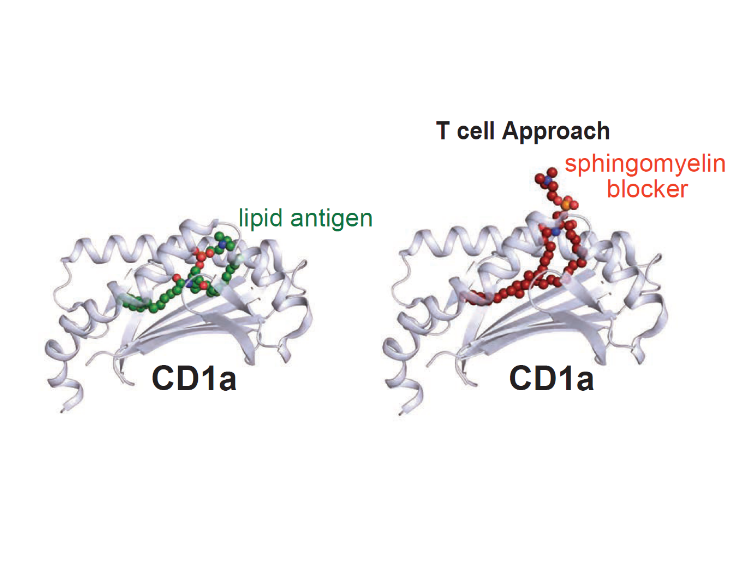
Figure 2: Lipid T cell blocker.
Building Upon Earlier Brigham Breakthroughs
The research from the Moody Lab builds on earlier methods that separate cellular lipids in one chromatographic system, which provided only a limited perspective. The new work details rules about the size, shape, and chemical content of the kinds of lipids that can bind CD1 and cause a T cell response—either activation or deactivation. It is the latest in a series of breakthroughs that date back to the 1990s, when Brigham scientists discovered that T cells can recognize lipid antigens.
While scientists have long known that T cells recognize antigens, until the 1990s, it was thought that these antigens were always peptides derived from proteins. Since lipids are not encoded by genes and are instead made by enzymes and form into membranes, they have entirely different functions and positions in the cell. The ability to measure many lipid antigens at one time will allow future researchers to cross-check any disease-related lipid of interest with the list of candidate lipid antigens from this map and potentially make connections to diseases.
“I applaud the tenacity of the researchers for building the critical mass of technology needed to develop this multi-stage system that allows large numbers of lipids to be identified, solved individually, and then grouped in patterns,” Dr. Moody says. “The Brigham provides an environment where physicians and scientists from differing fields can collaborate. This was a multidisciplinary effort that involved biophysical techniques related to mass spectrometry and biological techniques related to lipid chemistry. The lipids informed immunological outputs, and the mode of lipid recognition is proven through X-ray crystallography.”
Future Research With Clinical Implications
The Brigham’s approach to locating wet bench chemistry labs like Dr. Moody’s within hospital grounds fosters direct collaboration among physician-scientists and the ability to test molecular discoveries with clinical samples. As an example, Dr. Moody pointed to ongoing in vivo research conducted with Rachael A. Clark, MD, PhD, in the Brigham’s Department of Dermatology to detect human T cell responses and use a CD1a-binding lipid called sphingomyelin to block T cell inflammation that occurs in inflammatory skin diseases.
“Now that we have some general rules about what kind of lipids bind to CD1 and are displayed to T cells, we can focus on molecules that control the strongest T cell response,” Dr. Moody says. “The ability to block T cell response is particularly important in autoimmune diseases like atopic dermatitis, psoriasis, and Crohn’s disease. The therapeutic implications of CD1 blockers are quite promising.”