Elias, K, Ng, N, et al. (2023) Fertility restoration in mice with chemotherapy induced ovarian failure using differentiated iPSC. Lancet Biomedicine. In Press. Corresponding Author: R Anchan.
Researchers at Brigham and Women’s Hospital have demonstrated that mice with ovarian failure caused by chemotherapy can restore their fertility using induced pluripotent stem cells (iPSCs). Not only were the mice able to make functional eggs from the iPSCs, but those eggs developed into pups that could reproduce.
Although more research is needed before this approach can be evaluated in humans, this marks a big step toward reaching that goal. The study was published in July 2023 in Lancet Biomedicine.
“This research shows a novel, cell-based approach to treating infertility,” says senior author Raymond Manohar Anchan, MD, PhD, director of the Stem Cell Biology and Regenerative Medicine Research Laboratory at the Brigham. “It builds on earlier work from our lab that demonstrated that iPSCs can be used to generate ovarian and oocyte cell types.”
Using Stem Cells to Restore Ovarian Function
In the study, mice were treated with chemotherapy known to be toxic to the gonads.
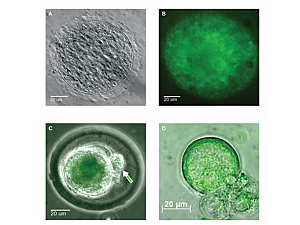
The iPSCs used in the research were derived from somatic ovarian granulosa cells. The cells were cultured in a three-dimensional environment that mimics the structure of the ovary, using human ovarian follicular fluid that otherwise would have been discarded by an in vitro fertilization clinic. The stem cells were engineered to carry a label with green fluorescent protein so their lineage could be traced after placing them in the mice.
“Because we labeled the stem cells, this gives us a high level of confidence that the developed eggs came from the stem cells that we introduced,” Dr. Anchan says. “These eggs clearly carry the label. Furthermore, we used short tandem repeat lineage training to confirm the stem-cell lineage of the resultant pups and litters.”
Nine of the mice that received iPSC transplants were crossbred with wild-type mice and were able to bear pups. Those pups were also bred to confirm their viability and fertility.
‘Epigenetic Memory’ Helps Stem Cells Form Ovarian Tissues
Dr. Anchan says another important finding from this work is that, in addition to being able to differentiate into egg cells, the stem cells can also make the reproductive hormones estrogen and progesterone. Additionally, the stem cells not only restored fertility to the ovary where they were placed but also boosted activity in the other ovary.
“We know this is the case because we get eggs in both ovaries, not just the one we injected,” Dr. Anchan says. “The stem cells are secreting something that helps the other ovary to recover some of its function. We confirmed this because not all the eggs contain the protein label.”
The researchers use the term “epigenetic memory” to describe the ability of cells taken from an organ to differentiate into the various cell types from that organ. Once placed into the ovaries of the mice, some of the cells were able to make follicles—the cavity where the eggs develop. “It’s intriguing because a single type of stem cell has the ability to do many different things,” Dr. Anchan adds.
Translating Ovarian Research to Human Cells
Ongoing experiments in Dr. Anchan’s lab are taking the next step toward translating these findings into approaches that would benefit human patients with ovarian failure. A common cause of this issue in women still of reproductive age is the chemotherapy regimen used to treat breast cancer. These drugs are known to deplete the follicles in the ovaries.
Dr. Anchan and his colleagues are already conducting research in cell cultures of human ovarian cells. They have shown that the iPSCs derived from discarded amniocytes can produce estradiol and progesterone when the same protocol is used. “Of course, we will need to come up with different ways to test the normal genetics of any eggs that may form before we begin to think about human trials,” he notes.
One thing that is still unknown is whether this approach would be effective only in patients who have lost ovarian function due to chemotherapy treatments or whether it would also work in those whose ovaries stop working prematurely due to a genetic condition. This is something the team plans to study, both in human cells and in animal models.
The authors also note that if this approach proves effective, it could provide the opportunity to develop a type of “autologous” hormone replacement therapy using patients’ own ovarian cells.