The American College of Rheumatology (ACR) hosts an annual Convergence conference with hundreds of sessions on cutting-edge topics in rheumatology. ACR Convergence 2022, referred to as “the world’s premier rheumatology experience,” was held November 10-14, 2022, as a hybrid meeting in Philadelphia, PA.
The Division of Rheumatology, Inflammation and Immunity at Brigham and Women’s Hospital played a prominent role in this year’s conference, with many members presenting at the meeting.
“The field of rheumatology is advancing rapidly in so many areas with the advent of new scientific technologies and their application to rheumatic diseases,” says Division Chief Ellen M. Gravallese, MD. “This tremendous progress was highlighted in the recent ACR conference, and our division was honored to help make it a success.”
Brigham physicians and scientists contributed over 120 lectures and poster presentations, including 10 invited lectures. Highlighted below are just a few of the meeting presentations from division faculty.
Tissue NK Cells and T Cells and Tissue Damage in Rheumatoid Arthritis and Systemic Lupus Erythematosus
It is known that T cells are important in rheumatoid arthritis (RA), but a new study by the Accelerating Medicines Partnership (AMP) RA/SLE consortium provides new insights into T cell populations in different individuals with RA. As part of this large collaborative study, Brigham researchers studied cells from over 70 synovial tissue biopsies from patients with RA using cutting-edge technologies, including single-cell RNA-seq and CITE-seq. They looked at the cellular composition of the inflamed synovial tissue and found that they fell into six categories, called cell type abundance phenotypes (CTAPs). Three of the six categories feature a large number of T cells. Surprisingly, three T cell-rich CTAPs were associated with three different kinds of T cells, namely peripheral helper and follicular helper CD4 T cells, GZMK+ CD8 T cells, and GZMB+ CD8 T cells. The cellular properties of these cells may help explain the characteristics of each of these subsets of RA.
Identifying Shared Genetic Architecture Between Rheumatoid Arthritis and Other Conditions: A Phenome-wide Association Study
Patients with RA commonly ask about the risk their family members have for developing RA or other related conditions. Researchers now have the tools to better answer this question with the availability of large biobanks and bioinformatics methods, enabling them to screen for associations between having genes for RA against about 1,800 conditions. In this study, Dr. Liao and her team observed that individuals carrying a higher burden of RA genetic risk alleles were at elevated risk for conditions such as hypothyroidism, type 1 diabetes, and vasculitis, with a reduced risk for celiac disease and multiple sclerosis. Additionally, for some conditions such as celiac, the lower risk is driven by a specific genetic region; thus, it matters which RA risk allele(s) were inherited.
Edmund L. DuBois Memorial Lecture: Exploring Immune System Phenotyping to Identify Novel Biomarkers
Dr. Rao presented on the ongoing efforts to use high-dimensional immunophenotyping of peripheral blood cells from patients with lupus to define a signature of immune dysregulation in lupus. Using mass cytometry and single-cell RNA-sequencing, researchers are identifying specific features of the activated immune response in patients with lupus, including the expansion of a T cell population discovered a few years ago called T peripheral helper cells. These features of immune dysregulation could hopefully be used to develop specific diagnostic tests for clinical use to detect and track immune system activation in patients with lupus.
The Accelerating Medicines Partnership: Lessons Learned and the Path Forward
The NIH-funded Accelerating Medical Partnerships (AMP) program has made major investments in understanding rheumatic diseases using high-dimensional molecular data, such as the application of single-cell technologies to inflammatory synovium from patients with RA. The AMP organized investigators to ensure patient recruitment, sample procurement, application of high dimensional technologies, and analysis of the resulting data. These data sets are large and complex and require cutting-edge computational methods to integrate different data types. In this presentation, Dr. Raychaudhuri described some of the key methods and algorithms that emerged through the work of this consortium.
Pre-existing RA Patients Initiating Checkpoint Inhibitors for Cancer Treatment: A Comparative Cohort Study Investigating RA Flare and Mortality
Patients with pre-existing RA who initiate immune checkpoint inhibitors (ICI) for cancer may be at risk for mortality, RA flare, and other immune-related adverse events (irAE). Dr. Sparks, Kaitlin McCarter, MD, and team found that patients with pre-existing RA initiating ICI for cancer had similar risks for mortality and severe irAE as non-RA comparators matched by age, sex, year, ICI target, and cancer type. Pre-existing RA was associated with a 72% higher risk for any irAE than comparators, but this was mostly due to RA flares that occurred in 48% that were typically mild. Pre-existing RA was not associated with severe irAE. Some irAE types, such as rash/dermatitis, endocrine, and hepatitis, were less commonly experienced among those with RA. This suggests that the immune system may be primed for RA flares after ICI or that the treatment of RA flares may mask or prevent other irAE types. These results suggest that pre-existing RA should not be considered a contraindication for ICI treatment for cancer.
Methodology and Item Considerations for CPPD Classification Criteria
Dr. Tedeschi and team presented a draft of the first-ever classification criteria for calcium pyrophosphate deposition (CPPD) disease, a common crystalline arthritis. The guidelines are currently in review by the ACR and the European Alliance of Associations for Rheumatology (EULAR). Dr. Tedeschi discussed the methods followed by an international, multidisciplinary team to develop a framework for classifying patients with CPPD disease to facilitate inclusion in research studies. The draft CPPD classification criteria framework achieved a high specificity (92.5%) and high sensitivity (99%) for CPPD disease in an independent validation cohort.
Single-Cell Spatial Transcriptomic Analysis Reveals Distinct Cellular Networks in RA Synovia
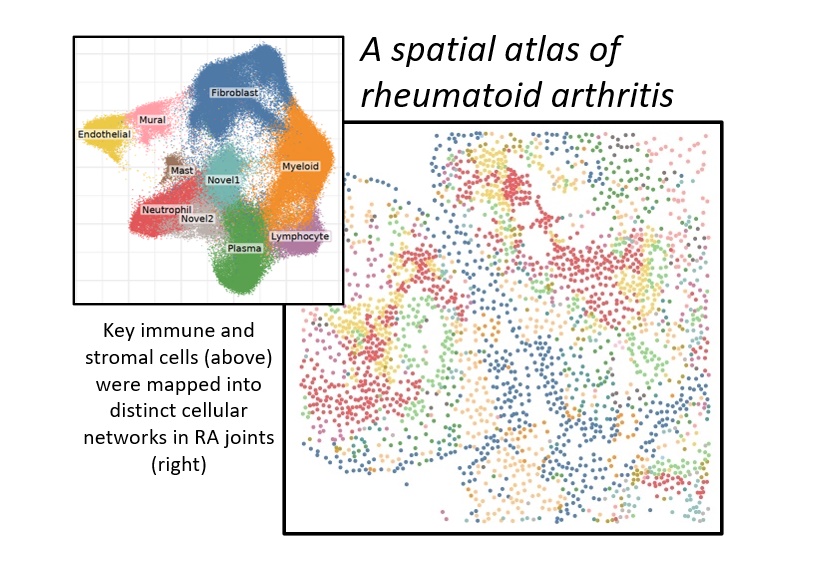
Chronic joint inflammation in RA arises from the interactions of many cell types. Single-cell transcriptomic profiling of joints has identified novel cell states and pathogenic pathways in RA. The ability to visualize cellular interactions directly in the joints of RA patients thus represents a major unmet need in research and an impediment to precision medicine.
Dr. Wei and Ilya Korsunsky, PhD, successfully applied a next-generation, single-molecule, spatial transcriptomic technology on joint tissue from RA patients. By integrating spatial transcriptomic data with single-cell transcriptomic profiles of joint tissue cells, the team identified 14 unique cellular niches compromised of distinct immune and stromal cell states. This approach is the first single-molecule, spatial transcriptomic atlas of RA joint tissue. The ability to visualize gene expression at a single-molecule resolution in RA joints adds new dimensions to our understanding of the pathological cellular networks of RA.
