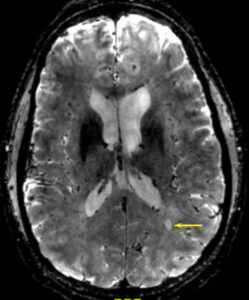
 From 7T MRI performed at Brigham and Women’s Hospital in Study Patient 1. This is a T2* gradient-echo sequence (0.8 mm isotropic voxels) showing a typical MS lesion (arrow) as oval, and bright, containing a central (dark) vessel.
From 7T MRI performed at Brigham and Women’s Hospital in Study Patient 1. This is a T2* gradient-echo sequence (0.8 mm isotropic voxels) showing a typical MS lesion (arrow) as oval, and bright, containing a central (dark) vessel.
During the COVID-19 pandemic, patients with multiple sclerosis (MS) and their clinicians have had questions and concerns about whether immunotherapies for MS could influence risk for infection or lead to an unfavorable outcome.
In the Journal of Neurology, Rohit Bakshi, MD, a senior neurologist at Brigham and Women’s Hospital, and international co-authors present the cases of five MS patients who developed COVID-19 infection while taking the oral disease-modifying therapy teriflunomide and continued taking the medication. All five patients had favorable outcomes, with their COVID-19 taking a mild course and without experiencing relapse of their MS.
“Managing MS during the COVID-19 pandemic has raised many questions,” said Bakshi, the corresponding author of the paper. “Our observations in these five patients suggest that teriflunomide may not need to be discontinued in patients with MS who develop an active COVID-19 infection. We also discuss potential direct anti-viral effects of teriflunomide.”
Investigating Teriflunomide’s Role in COVID-19 Outcomes
In the current international, multicenter study, Bakshi and co-authors, including lead author Amir Hadi Maghzi, MD, a clinical and research fellow in the Brigham’s Neurology Department, report on five patients, ranging in age from 52 to 79, who had been taking teriflunomide for at least six months. The patients continued their teriflunomide therapy after COVID-19 diagnosis and had self-limiting illness without experiencing MS relapse.
Treatment for MS usually requires long-term therapy, often with immunomodulating or immunosuppressing drugs. Teriflunomide modulates the immune response by selectively reducing the level of activated T and B lymphocytes without suppressing the body’s full immune response. One possibility, the authors write, is that teriflunomide could prevent an excessive immune response while maintaining an adequate defense against the virus. The authors also discuss pre-clinical data suggesting that the drug may reduce viral nucleotide synthesis in infected cells.
The case series was small, retrospective, open-label, uncontrolled, and non-randomized, and the authors state that future studies are necessary to understand what role, if any, teriflunomide therapy may play in COVID-19 infection since patient recovery may be unrelated to the treatment.
“A delicate balance may be necessary in the host immune response to successfully confront COVID-19 infection,” said Bakshi. “Additional studies are warranted to further understand the relationship between treatment with teriflunomide and outcomes for MS patients with COVID-19.”
Disclosures: Maghzi is supported by a clinician-scientist development fellowship from the National Multiple Sclerosis Society and American Brain Foundation. Bakshi has received consulting fees from Bayer, Biogen, BMS/Celgene, EMD Serono, Genentech, and Novartis, and research support from BMS/Celgene, EMD Serono and Sanofi-Genzyme.