Brigham and Women’s Hospital rheumatologist Deepak A. Rao, MD, PhD, was one of the leaders of a 2017 study published in Nature that identified a unique population of T cells found in high levels in the joints of people with rheumatoid arthritis (RA). These cells, which study investigators called “T peripheral helper (Tph) cells,” were shown to be particularly effective in stimulating B cell responses.
Dr. Rao has since been involved in efforts to pursue these cells as a potential therapeutic target in RA. During this time, many groups—including his own—have also identified Tph cells across multiple autoantibody-associated autoimmune conditions. They are particularly prominent in lupus, as Dr. Rao’s group showed in 2019 (published in JCI Insights).
More recently, Dr. Rao has set his sights on a different autoimmune condition: checkpoint inhibitor-associated arthritis. This is an inflammatory arthritis that develops after checkpoint blockade with immune checkpoint inhibitor (ICI) cancer therapies. The Brigham’s Division of Rheumatology, Inflammation, and Immunity has co-led a study to examine the rheumatologic manifestation of the disease, which affects about 5% of patients treated with anti-PD-1 therapy, a type of ICI.
“What we’ve tried to ask,” Dr. Rao says, “is to what extent does the immune response that develops after checkpoint blockade look like the immune response that you see in rheumatic diseases like RA or psoriatic arthritis?”
The Downside of ICI Therapies
The study, currently available as a preprint on bioRxiv, is a collaboration between the Brigham and a team at New York’s Hospital for Special Surgery (HSS) led by Anne R. Bass, MD, and Laura Donlin, PhD. The two institutions have collected synovial fluid and tissue samples from the joints of patients with checkpoint arthritis while also sharing the samples with each other and analyzing them together using mass cytometry and single-cell RNA sequencing.
Dr. Rao points out that though ICI therapies have revolutionized the treatment of advanced cancers, they also have a downside.
“These therapies can induce an immune response against the tumor, but they also induce unintended immune responses against a variety of organs,” he says. “This is an opportunity to study the development of an autoimmune disease as it starts with the initiation of ICI therapy.”
The investigators have found that after anti-PD-1 therapy, these patients do not develop large populations of Tph cells. This is a contrast with what is typically seen in RA.
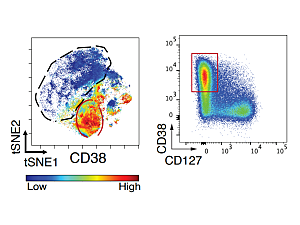
Another key finding: Rather than a high frequency of Tph cells, the investigators have seen a large population of a different T cell subset characterized by very high expression of a surface receptor called CD38.
“These CD38hi CD8+ T cells appear particularly prominently in checkpoint arthritis, really not so much in RA or psoriatic arthritis,” Dr. Rao says. “We’ve used RNA sequencing and functional studies to characterize these cells and try to understand their potential role in the disease. We’ve found that these cells look highly activated and inflammatory. They produce factors like TNF and interferon-gamma, and they look by their transcriptomes to be quite cytotoxic.”
Uncovering a Systemic Immune Response
In collaboration with Dr. Donlin, Brigham investigators have looked at the T cell receptor repertoires of this same T cell subset in both the joints and the blood. An interesting development occurred at HSS, where the team was able to collect synovial tissue from both knees of a patient with advanced cancer. The patient had had an excellent tumor response to ICI therapy but then developed a destructive inflammatory arthritis that required bilateral knee replacements. Study investigators detected clones of the same T cells in both knees.
“This suggests the immune response induced by ICI therapy is a systemic one that’s happening throughout the body and involving multiple joints in a similar way,” Dr. Rao says. “One can also find some of those same clones that are enriched in the joints also circulating in the blood, suggesting that those cells are potentially migrating from joint to joint.”
In addition, investigators have looked further at blood samples from a broader set of patients who developed immune-related adverse events after checkpoint blockade. According to Dr. Rao, they have seen an increased frequency of CD38hi CD8+ T cells in the peripheral blood of patients with checkpoint arthritis. “This suggests we may be able to measure things in the blood to help assess the extent of immune activation in these patients,” he says.
Another interesting takeaway for Dr. Rao concerns the arthritis caused by the immune response in checkpoint blockade, which has distinct features from what is usually observed in RA and psoriatic arthritis. One such feature is that the cells coming from patients with checkpoint arthritis express an interferon signature, a feature typical of lupus.
“This is sort of an unexpected finding because we don’t see a strong interferon signature in RA or psoriatic arthritis, and there are very few cases of checkpoint inhibitor-induced lupus,” Dr. Rao says. “This checkpoint inhibitor-associated arthritis is an unexpected meld of an inflammatory arthritis with an interferon signature.”
Interferons have not been a major point of focus in either RA or psoriatic arthritis therapeutics. However, citing recent phase 3 clinical trial data, he notes that interferon blockade has emerged as an efficacious therapy in lupus.
“Our findings raise the prospect of targeting interferons as a potential therapy to dampen the adverse immune responses induced by checkpoint blockade, with the consideration that you must carefully manage the potential risk of interfering with the anti-tumor response,” Dr. Rao says.