By taking the brakes off the immune system and allowing it to attack cancer cells, checkpoint inhibitors have spurred significant advances in the treatment of many people with cancer over the past decade. But for patients who do not respond to these drugs — still the majority of people with cancer — alternative interventions are needed.
A team of investigators from Brigham and Women’s Hospital and MIT is using the power of nanotechnology to develop an entirely new approach for boosting the immune response to cancer. Through the use of field-emission scanning electron microscopy (FESEM), they have learned that cancer cells actually steal mitochondria from T cells — an action that both strengthens the cancer cells and weakens the immune response.
“Nanotechnology is helping us to better understand important basic biological functions,” says Shiladitya Sengupta, MS, PhD, co-director of the Brigham’s Center for Engineered Therapeutics and the study’s senior author. “If we could develop a drug to inhibit this hijacking of mitochondria, it could make immune checkpoint therapy much more effective. That’s what we’re now trying to do.”
Dr. Sengupta and team’s latest study was published in November in Nature Biotechnology.
Observing the Transfer of Mitochondria
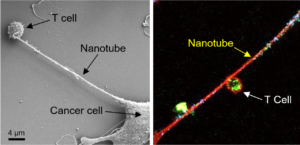
To study the interactions between cancer cells and immune cells, Dr. Sengupta’s team co-cultured breast cancer cells with different types of immune cells, including T cells. When they looked at the cells using FESEM, they saw that tube-like filaments were connecting the two types of cells. “The cancer cell sends out these nanotubes, which are almost like tentacles,” he says.
When researchers zoomed in further, they noticed there were small particles along the length of these filaments. Suspecting the particles were mitochondria, they labeled these organelles with a fluorescent green dye that bonds to mitochondrial proteins. They then were able to observe the mitochondria being pulled out of the T cells, traveling the length of the nanotubes and being incorporated into the cancer cells.
Further analysis of the metabolic functions of both the immune cells and the cancer cells confirmed that the shift in mitochondria from one cell to the other was affecting cell function.
Look for Drug Strategies to Protect Immune Response
Next, the researchers tested whether they could intervene and prevent the cancer cells from hijacking mitochondria. In mouse models of breast cancer, they injected a drug that blocks the formation of these filaments. When they combined this agent with immune checkpoint inhibitors, they saw a significant reduction in tumor growth in the mice.
Dr. Sengupta’s team is now working with other investigators to develop next-generation filament-inhibiting agents that are more drug-like and potentially could be developed for evaluation in clinical trials.
“The implication of this work is that if we can prevent the mitochondrial hijacking from happening, essentially preventing the T cell’s batteries from being stolen by the cancer cells, then the immune cell retains the energy to attack the cancer cell,” he explains.
Dr. Sengupta expects this approach could be successful for a number of different solid tumors. “We’ve observed this behavior in lung cancer, breast cancer, melanoma and many other tumor types,” he says. “We believe this could be part of a broad strategy for making immunotherapy more effective.”
Brigham and Women’s Hospital is committed to fueling life-saving advancements in cancer research and care. Learn about the Cancer Research Center at the BWH Brigham Research Institute.