 For most chronic diseases, improvements in care over the past few decades have resulted from early interventions that prevent disease progression. Brigham and Women’s Hospital investigators, along with collaborators at other institutions around the world, are applying a similar approach to the early detection and prevention of Alzheimer’s disease (AD).
For most chronic diseases, improvements in care over the past few decades have resulted from early interventions that prevent disease progression. Brigham and Women’s Hospital investigators, along with collaborators at other institutions around the world, are applying a similar approach to the early detection and prevention of Alzheimer’s disease (AD).
Some of this research is being conducted through the Davis Alzheimer Prevention Program (APP), launched in July 2020. Brigham neurologists Reisa A. Sperling, MD, MMSc, and Dennis J. Selkoe, MD, are co-leading the program.
“The overall goal of the Davis APP is to accelerate finding a successful preventative treatment for AD,” said Dr. Sperling, an internationally recognized expert in AD and director of the Brigham’s Center for Alzheimer Research and Treatment. “To help reach this goal, we want to select drugs for clinical trials that are more likely to work, as well as more quickly eliminate those that don’t. We also aim to develop more sensitive ways to detect changes in the brain — both with biomarkers in the blood and imaging methods.”
She added that in addition to accelerating work in both of these areas, another important initiative under the Davis APP is to increase the diversity of patients in AD clinical trials. This includes a focus on outreach and community partnerships.
Launching Innovative AD Clinical Trials
Dr. Sperling also serves as principal investigator for the National Institutes of Health-funded Alzheimer’s Clinical Trials Consortium (ACTC), a network of 35 sites across the country focused on finding new ways to prevent and treat AD.
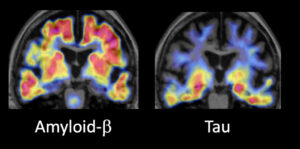
Two trials recently launched under the ACTC are AHEAD 3 and AHEAD 45. Both are investigating the effectiveness of the antibody drug BAN2401 (lecanemab) to slow or stop the accumulation of ß-amyloid in individuals who are at greatest risk of developing AD. The risks are determined by age, family history and PET scans of the brain.
“The AHEAD studies are some of the first to use targeted dosing, where we screen people who are cognitively normal and assign them to a trial based on the amount of ß-amyloid seen on their PET scan,” Dr. Sperling said. “This is a way of offering more personalized medicine.”
In the AHEAD 3 study, patients with low levels of ß-amyloid get monthly dosing of the drug. The AHEAD 45 study includes people with higher levels of ß-amyloid, even though their cognitive function is normal. “With this group, we want to be more aggressive in trying to knock their ß-levels down,” Dr. Sperling said. These trial participants get doses every two weeks for the first two years, then a monthly maintenance dose.
The AHEAD trials were launched last summer in the United States, funded as a public-private partnership with the National Institutes of Health. The AHEAD Study opened its first site in Japan this fall, and up to 100 sites around the world will participate in the coming years.
Different Approaches to Drug Development
As a neurologist who treats AD patients in all levels of cognitive decline, Dr. Sperling recognizes the need to develop better treatments for active disease and preventative drugs. She also has witnessed AD in her own family, with her father and grandfather.
“For people who already have clinical symptoms of Alzheimer’s, I think we will need to do something more aggressive,” she noted. “That will likely be a combination of drugs that target ß-amyloid and drugs that target tau.”
Dr. Sperling stressed the importance of good trial design for some of these combination approaches. “We are now working on how to combine anti-amyloid antibodies with anti-tau drugs and how to measure potential synergistic effects with tau PET imaging,” she said.
Better Screening Methods Support Clinical Trials
Developing and evaluating new interventions to prevent AD progression as well as new treatments to alleviate AD symptoms will require better assays to predict cognitive decline. In a recent study published in Nature Communications, Dr. Sperling co-led a team that looked at a blood test that measures levels of N-terminal fragment of tau (NT1, a protein secreted by neurons in response to ß-amyloid pathology). The test was evaluated in participants in the Harvard Aging Brain Study, a group of cognitively normal older adults who are being followed over time.
The study analyzed the predictive value of NT1 in 236 study participants who were cognitively normal and followed them for an average of five years. The researchers found that participants whose blood samples had higher levels of NT1 at the beginning of the testing period had a higher risk of advancing to mild cognitive impairment. Imaging showed that higher levels of NT1 were also associated with more ß-amyloid plaques and greater accumulation of tau tangles.
“We don’t know yet how dynamic levels of NT1 are in the blood over time, but we know that NT1 goes up pretty early before people have cognitive symptoms,” Dr. Sperling said. “We have high hopes that this may work as an indicator of whether drugs are working, as we move into more prevention trials.”