The American College of Rheumatology’s annual Convergence conference, which took place Nov. 5–9, featured significant contributions from Brigham and Women’s Hospital physicians and scientists. The tally included over 120 abstracts: two plenary presentations, 36 oral presentations, one late-breaking poster and 86 poster presentations.
Ellen M. Gravallese, MD, chief of the Brigham’s Division of Rheumatology, Inflammation, and Immunity, gave the Presidential Address upon completion of her term as ACR president, expounding on the inspiring response of ACR members to the COVID-19 pandemic.
Dr. Gravallese summarized lessons learned during the pandemic, including the importance of rheumatologists as experts in immunology, the optimal use of telemedicine and virtual education and the importance of organizational change to allow societies and institutions to respond more nimbly to crises. “If we heed these lessons and implement change thoughtfully, rheumatology will enter a new era as an even more essential and highly sought-after specialty,” she said in her speech.
Two long-time Brigham rheumatologists also received prestigious honors. Michael B. Brenner, MD, was named an ACR Master, which recognizes “outstanding contributions to the ACR and the field of rheumatology through scholarly achievement and/or service to their patients, students, and profession.” In addition, Daniel Hal Solomon, MD, MPH, was given the Excellence in Investigative Mentoring Award, which honors “contributions to the rheumatology profession through outstanding and ongoing mentoring.”
For the first time, ACR Convergence 2020 was a virtual event. Drs. Gravallese and Solomon lamented the inability to interact directly with fellow participants but noted an upside: Over, 14,000 people from 111 countries attended at least some portion of the conference, compared with the typical 9,000 to 10,000 attendees.
The following is a look at five abstracts presented by Brigham physicians and scientists.
Identifying Immune Cells That Cause Toxicities of Checkpoint Inhibitor Therapy
Runci Wang, MD, PhD; Karmela Kim Chan, MD; Amy Cunningham-Bussel, MD; Gregory Vitone; Aidan Tirpack; Caroline Benson; Gregory Keras; Anna Helena Jonsson, MD, PhD; Michael Brenner, MD; Laura Donlin, PhD; Anne Bass, MD; Deepak A. Rao, MD, PhD
Therapies to induce immune responses against tumors, such as anti-PD-1 antibodies, have revolutionized the treatment of many cancers. These “checkpoint inhibitor” therapies can unleash a powerful immune response against tumors but can also induce unintended autoimmune responses, including inflammatory arthritis.
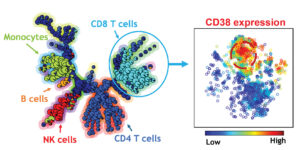
Dr. Rao led a team that used mass cytometry immunophenotyping to study the immune cells that accumulate in joint fluid of patients who develop inflammatory arthritis following anti-PD-1 therapy. The study revealed a unique population of CD38hi CD8+ T cells that accumulated in the joints of patients with inflammatory arthritis following anti-PD-1 therapy. These cells are highly activated and are equipped to kill target cells.
“We hope that by understanding the similarities and differences between immune cells in different types of inflammatory arthritis, including checkpoint inhibitor-associated arthritis, we can identify specific signals that block the key pathologic signals in each condition,” Dr. Rao said.
Challenges and Opportunities in Using Machine Learning & Natural Language Processing to Define Eligibility Pool
For many clinical trials in rheumatology, identifying eligible patients is a labor-intensive and rate-limiting step. The increasing availability of detailed electronic health record data, along with methods such as machine learning and natural language processing (NLP), provide an opportunity to streamline our ability to find these patients.
Dr. Liao’s team developed an algorithm using machine learning and NLP that reduced the number of charts requiring review by 40–45 percent compared to reviewing any subjects with an ICD code for rheumatoid arthritis (RA). Importantly, exceedingly few potentially eligible patients were eliminated by screening using this algorithm.
Decoupling Inflammation and Bone Loss in Rheumatoid Arthritis via Schnurri-3
Zheni Stavre, MD; JungMin Kim, PhD; Jae-Hyuck Shim, PhD; Ellen M. Gravallese, MD
RA is a chronic inflammatory disease that leads to local and systemic bone loss. TNF is a key mediator of bone loss, not only through promotion of osteoclasts (bone-resorbing cells), but also through inhibition of osteoblast (bone-forming cells) function.
This team targeted schnurri3 (Shn3) — a large, intracellular protein upregulated by TNF and IL-17A and known to be a suppressor of osteoblast activity — in specific bone cells in several mouse models of RA and determined its therapeutic potential in RA-induced bone loss. The work provided promising evidence that targeting this pathway could be a novel therapeutic strategy for both systemic and joint-based RA-induced bone loss.
Single-Cell Profiling of Synovial Stromal Cells Reveals an Angiocrine Endothelium in Rheumatoid Arthritis
Kevin S. Wei, MD, PhD; Ilya Korsunsky, PhD; Jennifer L. Marshall, PhD; Gerald F.M. Watts, MSc; Trinn Major, PhD; Zhu, MSc; Yuhong Li, MSc; Christopher Buckley, MBBS; Soumya Raychaudhuri, MD, PhD; Michael B. Brenner, MD
These investigators recently reported that a key step in pannus formation and fibroblast expansion in RA is driven by endothelial-derived Notch signaling. Their observation suggests that synovial endothelial cells, rather than being passive conduits of blood flow, orchestrate tissue remodeling through secretion of paracrine morphogenic signals.
In this study, the team performed single-cell transcriptomic analysis (scRNAseq) of synovial vasculature to characterize vascular remodeling at a single-cell level. These analyses identified enrichment of Notch receptor activation among arterial endothelial cells and a subset of capillaries, implicating Notch signaling during vascular remodeling in RA. The study highlights the power of single-cell transcriptomics in identifying cellular and molecular changes in organ tissues affected by inflammatory diseases.
The Impact of an Integrated Care Management Program on Acute Care Use and Social Determinants of Health for Individuals With Lupus
Jessica N. Williams, MD, MPH; Kreager Taber; Candace H. Feldman, MD
A nurse-led integrated care management program (iCMP) aims to coordinate medical care and to uncover and address social determinants of health-related needs for medically and psychosocially complex patients at high risk for frequent emergency department visits and hospitalizations.
Investigators examined whether iCMP was effective at decreasing the incidence rates of ED visits, hospitalizations and outpatient appointment no-shows. They also aimed to understand whether social determinants of health-related needs such as food insecurity, housing instability and financial constraints were prevalent among patients with lupus enrolled in iCMP. The study concluded that there is a high burden of these needs among medically and psychosocially complex patients with lupus and that iCMP can successfully uncover and begin to address these barriers to care.
“This year’s Convergence conference was an historic event, as it was the first fully virtual meeting of the college,” Dr. Gravallese concluded. “The Brigham’s Division of Rheumatology, Inflammation, and Immunity provided an important scientific presence at the meeting, and many division members served as moderators and session leaders, contributing significantly to the meeting’s impact and success.”