Dong et al., Genome Sequencing Explores Complexity of Chromosomal Abnormalities in Recurrent Miscarriage, The American Journal of Human Genetics (2019)
For couples with recurrent miscarriage (RM), the condition remains unexplained in about 40 to 60 percent, even after costly testing. Chromosomal abnormalities—rearrangements of large chunks of DNA—in the genomes of one or both individuals trying to conceive are thought to be among the major genetic causes of RM. But routine chromosome analysis (karyotyping) can currently detect these abnormalities in only about 1 in 50 couples.
A new study by investigators from Brigham and Women’s Hospital, Shandong University and The Chinese University of Hong Kong used low-pass genome sequencing (GS) to look for chromosomal abnormalities in couples with RM. Low-pass GS revealed additional chromosomal abnormalities in more couples than traditional testing, increasing detection to 1 in 9 couples with RM. Karyotyping is a low- resolution technique that offers a level of detail similar to a bird’s-eye view of the ground below, whereas genome sequencing is a high-resolution technique (at nucleotide level) comparable to seeing the ground with a microscope.
“It’s often difficult to know how to treat or counsel couples when the cause of their infertility remains unknown. There are couples who have chromosomal rearrangements that can’t be seen by classical methods. The technique we’ve used here increases the number of couples who we can detect who are at risk for having miscarriage,” said co-author Cynthia Morton, PhD, director of cytogenetics at Brigham and Women’s Hospital.
This is particularly important because the information is useful for determining who would benefit from costly preimplantation genetic testing (PGT). “Our results suggest that applying low-pass GS could help identify a larger subgroup of patients at increased risk of subsequent miscarriage who might take advantage of preimplantation genetic testing,” said Morton.
A Fundamental Role for Genetics to Help People With Infertility
Building on a previous collaboration, researchers from Brigham and Women’s worked with co-corresponding author Kwong Wai Choy, PhD, and his graduate student and first author Zirui Dong, PhD, at The Chinese University of Hong Kong to explore further low-pass GS as a tool in clinical cytogenetics. With co-corresponding author Zi-Jiang Chen, MD, PhD, at Shandong University, the investigators analyzed samples from 1,090 couples who had two or more pregnancy losses.
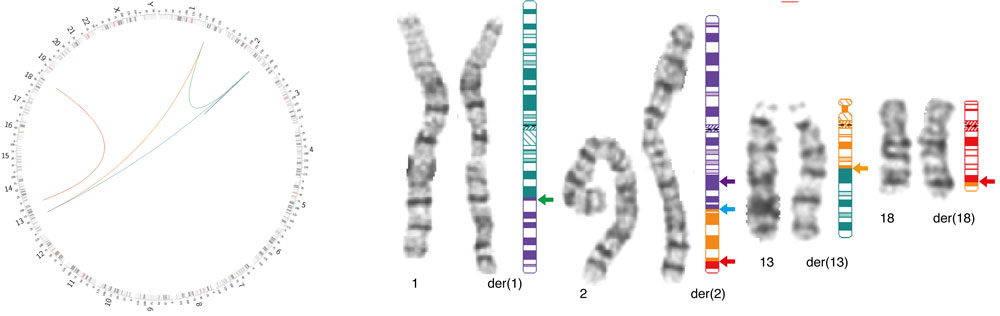
Couples were enrolled from RM clinics at the two universities. Investigators performed chromosome analysis and low-pass GS. The team detected 127 chromosomal abnormalities using low-pass GS, accounting for approximately 12 percent of the couples and about 50 more couples than traditional chromosomal analysis detected. The international team published “Genome Sequencing Explores Complexity of Chromosomal Abnormalities in Recurrent Miscarriage” in The American Journal of Human Genetics in late 2019.
This new finding of a higher therapeutic yield with genomic sequencing (approximately 12 percent, compared to 2 percent with karyotyping) has implications for the value of testing, the researchers said.
Co-author Raul E. Piña-Aguilar, MD, a postdoctoral fellow in the Morton lab, described the results as “a perfect example of the fundamental role that genetics can serve to help patients with infertility. We have found that additional RM couples have chromosomal rearrangements. Undoubtedly, these couples are the ones who will benefit most from personalized intervention.”
The new study is an extension of work in Morton’s lab to identify chromosomal disruption in a variety of clinical syndromes. Low-pass genome sequencing, which has been used in her lab for 20 years to decode the genome, now can be done at a cost and speed that enables it to be considered for use in widespread testing, Piña-Aguilar said.
For the study, a customized sequencing and interpretation pipeline was developed to identify chromosomal rearrangements and deletions/duplications with confirmation by fluorescence in situ hybridization, chromosomal microarray analysis and PCR studies. Currently, low-pass GS is used primarily for research and is available in the United States in only in a small number of clinical laboratories. In the next 3 to 5 years, genome sequencing for chromosomal abnormalities is likely to become more widely available.
Illuminating Which Couples May be Candidates for PGT
Couples with known genetic abnormalities would benefit most by PGT, a costly step that can add $20,000 or more to the patient’s expenses in addition to the cost of in vitro fertilization (IVF), the Brigham authors said.
“This is clinically relevant because couples with a genetic abnormality are not like other couples with infertility,” Piña-Aguilar said. “If the parents have a chromosomal abnormality, we know the embryo is at increased risk to be abnormal, leading to miscarriage or effects on the baby.”
“This opens up a way to manage couples differently,” Morton added. “Until now many of these patients were not even recognized. It illuminates which couples have a risk, and there’s something we can do about it.”
Current recommendations for genetic study of patients with RM vary internationally. The American Society of Reproductive Medicine (ASRM) recommends parental karyotyping but does not recommend testing the products of conception. Within the field worldwide, there has been a lack of consensus as to whether the therapeutic yield of various screenings was high enough to justify their costs and differing views of the likelihood that chromosomal abnormalities in embryos could self-correct.
Morton said it is reasonable to perform karyotyping in couples struggling with RM, while the rationale and availability of genomic screening expand for those with chromosomal abnormality.
Looking ahead, the Brigham researchers anticipate a single genome sequencing test that eventually could help discern a genetic reason for infertility, perform carrier screening and also screen for other chromosomal abnormalities.
“This is opening a door to do more genetic screening in OB/GYN,” Piña-Aguilar said.