Although surgery and radiation provide good disease control for localized prostate cancer, developing long-lasting approaches for treating metastatic disease has been challenging because resistance to androgen deprivation therapy (ADT) frequently develops.
In the past decade, next-generation antiandrogen drugs have shown efficacy against castration-resistant prostate cancer. But even with those drugs, most tumors eventually develop resistance. There continues to be an urgent need for understanding the mechanisms that contribute to castration-resistant prostate cancer and identifying other targets within the androgen receptor (AR) pathway for treatment.
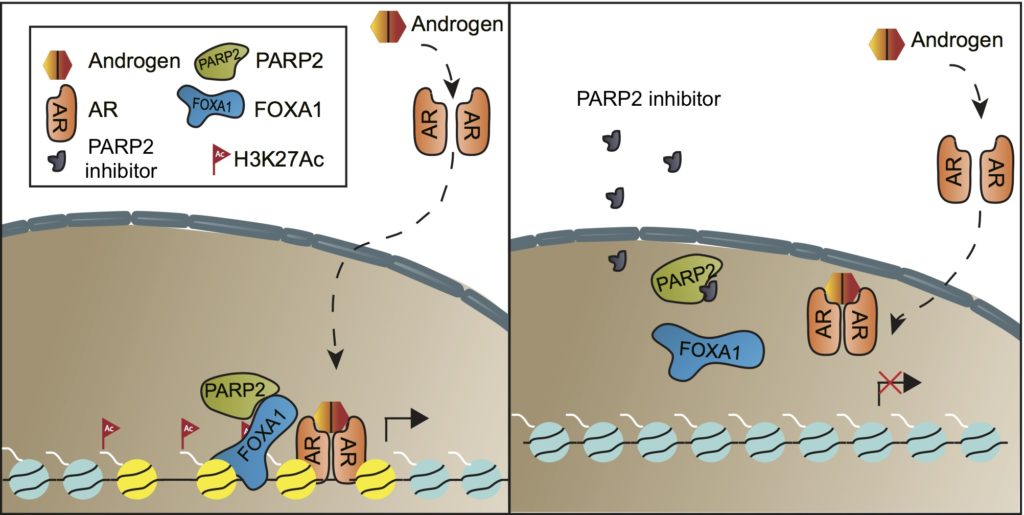
In a study published in July 2019 in the Proceedings of the National Academy of Sciences (PNAS), investigators from Brigham and Women’s Hospital and the Dana-Farber Cancer Institute reported a new approach that appears to be effective in preclinical models. It entailed blocking interactions between two proteins: FOXA1 and PARP-2.
“We know that the signaling and function of the AR is dictated by FOXA1,” said Li Jia, PhD, director of urology research, surgery, at the Brigham, who was a corresponding author on the PNAS paper. “Studies have shown that FOXA1 is a principle oncogene in prostate cancer. This makes it a very attractive target.”
Taking Aim at an ‘Undruggable’ Target

Since FOXA1 is transcription factor, it is widely considered to be undruggable. “But in this study, we actually found that PARP-2 interacts with FOXA1 and enhances its function. By blocking PARP-2, you can shut down AR signaling,” Dr. Jia said.
Drugs called PARP inhibitors have garnered a lot of attention in recent years. They work by preventing cells from utilizing DNA repair pathways, and this prevention leads to cancer cell death. Several of these drugs are already approved for ovarian and breast cancers driven by mutations in DNA repair genes, including BRCA1 and BRCA2. They are also showing promising results for other cancers driven by DNA repair mutations, such as prostate and pancreatic cancer.
“However, these drugs largely target PARP-1 instead of PARP-2,” said Adam Stuart Kibel, MD, chief of the Brigham’s Division of Urology and the study’s other co-corresponding author. “Rather than playing a role in the DNA repair pathway, PARP-2 is a critical component of the AR transcriptional machinery. By finding a way to disrupt FOXA1 function, we now have a therapy that will kill the cancer cell via an alternative mechanism.”
Importantly, PARP-2 is found at much higher levels in prostate cancer tumors than in benign prostate tissue, enhancing its appeal as a target.
Developing a New Treatment Approach
The compound tested in the PNAS study was originally identified as a tool to study PARP-2 biological functions. However, the team’s analyses revealed that it very specifically inhibited AR signaling. This led to the discovery of a new therapeutic strategy.

“This compound worked in cell lines and in animal models, but now we need to develop a drug that is more potent and specific at hitting the target and can be tested in clinical trials in patients,” Dr. Kibel said.
The investigators say they don’t yet know if a small-molecule approach or a genetic approach will ultimately prove more effective in blocking the AR pathway. But they are working with chemists at Dana-Farber as well as the University of Massachusetts, Boston, to look for new approaches.
Dr. Jia said that pharmaceutical companies have also shown interest in this pathway. In addition to looking for better drug candidates, the researchers will continue to do studies in the lab focused on better understanding of the role of PARP-2 in prostate cancer.
“Because tumors become resistant even to the newest drugs, it’s important to continue to identify additional targets,” Dr. Kibel said.