Patients with RA and OA all experience chronic pain, but safe options for analgesia are limited. While NSAIDs (selective and non-selective) are commonly used in rheumatology, they can cause major toxicity. Improving the risk/benefit ratio requires a more precise understanding of risk. This study, published in Arthritis & Rheumatology, was undertaken to derive and validate a risk score for major toxicity among NSAID users enrolled in a prior randomized controlled trial.
We used information from the PRECISION trial, a large randomized controlled trial that compared the safety of three NSAIDS (celecoxib, naproxen or ibuprofen) at typical dosages. In our study, patients enrolled in PRECISION who had known cardiovascular disease or risk factors as well as OA or RA were divided into derivation and validation cohorts. The risk score was designed to predict the 1‐year occurrence of major toxicity among NSAID users, including major adverse cardiovascular events, acute kidney injury, significant gastrointestinal events, and mortality. Variables significantly associated with major toxicity were candidates for inclusion in the final risk score model. After derived models were found to have a similar model fit in the validation set, the cohorts were combined and a risk score calculated. The study led by Daniel H. Solomon, MD, MPH, Chief of the Section of Clinical Sciences in the Division of Rheumatology at Brigham and Women’s Hospital, was done with collaborators from the Cleveland Clinic Foundation.
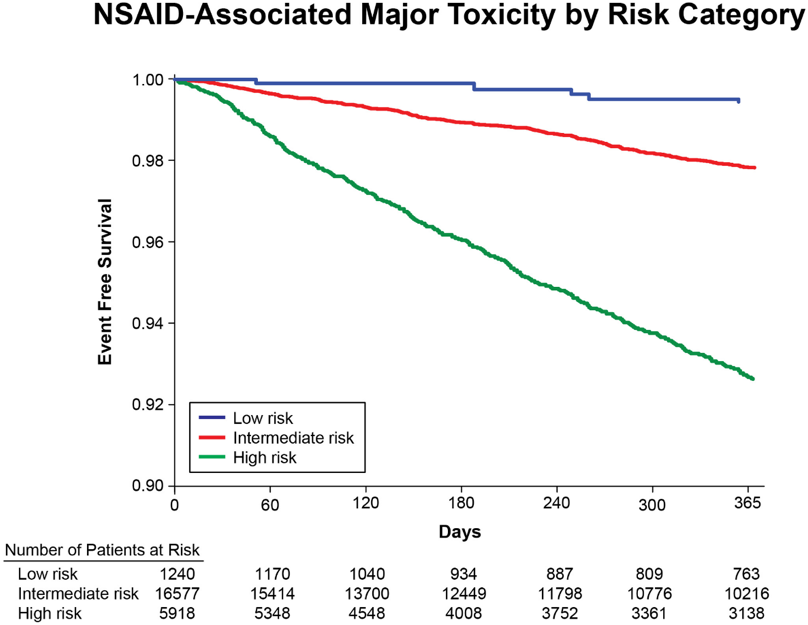
In the derivation cohort, significant variables included age, male sex, history of cardiovascular disease, hypertension, diabetes mellitus, tobacco use, statin use, elevated serum creatinine level, hematocrit level and type of arthritis. The AUC was 0.73 in the validation cohort and 0.71 in the total cohort, and the model was well calibrated. Of the total population with complete data (n = 23,735), 5% had a predicted 1‐year risk of major toxicity of <1%, 69% had a predicted risk of 1–4%, and 27% had a predicted risk of >4% (see Figure).
The risk score accurately categorizes the 1‐year risk of major toxicity among NSAID users and may be useful in identifying patients who can safely use these agents. External validation is underway and further refinement of the intermediate (1-4%) risk group is ongoing. If the risk score is found to be valid in external populations that may be more heterogeneous in characteristics, the risk of major NSAID toxicity can be predicted using simple clinical factors that should allow for more personalized decision-making regarding initiation of NSAIDs.