 For decades, the medical and scientific community has looked for ways to repair damaged vocal cords through injectable agents. Current best practices involve the use of carboxymethylcellulose, hyaluronic acid or calcium hydroxylapatite (CaHA). However, these injectable agents do not provide permanent effects and typically carry the additional burden of requiring two people to perform the medialization procedure, thus adding to their cost.
For decades, the medical and scientific community has looked for ways to repair damaged vocal cords through injectable agents. Current best practices involve the use of carboxymethylcellulose, hyaluronic acid or calcium hydroxylapatite (CaHA). However, these injectable agents do not provide permanent effects and typically carry the additional burden of requiring two people to perform the medialization procedure, thus adding to their cost.
A new injectable agent and injection device currently being investigated may eliminate these and other obstacles, according to a study published in The Laryngoscope. A novel vocal fold injectable that incorporated silk proteins was the brainchild a decade ago of Thomas L. Carroll, MD, of the Brigham and Women’s Hospital Division of Otolaryngology. A silk protein particle suspended in hyaluronic acid (Silk-HA) was subsequently developed by Medford, Massachusetts-based Sofregen Medical Inc. Carroll and Sofregen began a collaboration in 2016 to scientifically evaluate the novel material and co-authored the study.
In this small canine study, Silk-HA was found to provide medialization of the right vocal fold for a minimum duration of six months. It also remained at the injection site with no signs of migration. CaHA-based injectable agents unexpectedly migrated to tissues outside of the voice box and to tissues outside the neck, including lymph nodes. Such migration is likely to explain why surgeons occasionally encounter inflammation and other side effects with CaHA, as well as the need to perform unintended touch-up procedures.
“The Silk-HA particles are less likely to migrate because they are about 10 times the size of CaHA particles,” Dr. Carroll explained. “At the same time, it can be delivered through a small gauge needle, making it easy to inject.”
Dr. Carroll also noted that unlike stiff, solid CaHA particles, Silk-HA is more comparable to human tissue in terms of how it affects the deep vocal fold’s pliability. Silk-HA particles are also porous. This feature promotes collagen in-growth, which leads to the potential for permanent fibrosis, as demonstrated in a previous porcine study conducted by Dr. Carroll and colleagues at Sofregen, and published in the Journal of Voice.
“Even if the silk implant does not remain forever, there is potential for a lasting, long-term effect with Silk-HA,” Dr. Carroll said.
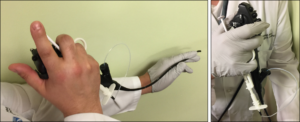
In addition to creating the new agent, Dr. Carroll co-developed a custom catheter-guided endoscopic delivery device in conjunction with Sofregen biomechanical engineers. The catheter, designed specifically for laryngoscopic delivery, can be inserted through an internal or external sheath channel of a flexible laryngoscope for more precise material placement by a single operator in the clinic or at the bedside under local anesthesia.
Dr. Carroll described the combination of the Silk-HA injectable agent and the injection mechanism as a cost-effective advance in vocal fold injection augmentation. “It is an ‘off-the-shelf’ solution that can be administered easily in an outpatient clinic by a single ENT generalist to provide permanent vocal fold medialization,” he said.
“This work is a great example of how physicians and surgeons at the Brigham can partner with industry to push the envelope in terms of technological advances that will impact the ways in which we can cost-effectively serve our patients,” Dr. Carroll added. “It’s a wonderful reflection of the incredible environment of innovation and engineering we have here at the Brigham.”