A single genetic variant in PTPN22 has been known for over a decade to convey risk for rheumatoid arthritis (RA), but exactly how remains a mystery. Led by rheumatologist and researcher Dr. I-Cheng Ho, investigators at BWH are using the JBC to understand PTPN22 as a regulator of citrullination, the post-translational protein modification of arginine that plays a leading role in RA biology.
Having observed previously that PTPN22 inhibits the citrullinating enzyme PAD4,1 and that individuals carrying the risk variant of PTPN22 exhibited enhanced citrullination of peripheral blood mononuclear cells (PBMCs), they hypothesized that hypercitrullination could be an early pathogenic step in RA development. Indeed, they found hypercitrullination in PBMC not only in patients with early confirmed RA but also in healthy individuals at high RA risk – that is, first degree relatives of RA patients and individuals with anti-citrullinated protein antibody (ACPA) positivity. This phenotype was related to downregulation of PTPN22, even in individuals lacking the PTPN22 risk variant, and translated into enhanced production of pro-inflammatory cytokines including IL-2 and IL-17. Thus, hypercitrullination and associated functional changes seem to be a key early step in the development of RA (even in patients who are not ACPA positive), defining a “molecular signature” of pre-clinical and early RA, as published in JCI Insight.2
These findings provided the preliminary data for a new study enabling recruitment of human subjects using the JBC. Dr. Ho and his team were interested in dose effects among healthy individuals related to having zero, one, or two copies of the PTPN22 risk variant. JBC Young Investigator Dr. Hui-Hsin Chang, a member of Dr. Ho’s laboratory, submitted a JBC Microgrant that was funded to prospectively recruit individuals based on PTPN22 genotypes (homozygous controls, heterozygous cases, and homozygous cases). The Partners Biobank consists of >80,000 patients recruited from BWH and Massachusetts General Hospital who have donated biospecimens linked to their medical records, together with consent for re-contact for research. Over 25,000 of these individuals have been genotyped, and after appropriate regulatory approval, these patients can be called back in to provide a sample of blood. The JBC Human Biosamples Core team, led by Drs. Elizabeth Karlson and Jeffrey Sparks of BWH, coordinated with the Partners Biobank to identify healthy individuals by PTPN22 genotype. The JBC assisted Dr. Ho in preparing and submitting the required Institutional Review Board (IRB) documents, in receiving approval from the Partners Biobank, and in obtaining gift cards and parking vouchers for subject remuneration.
After IRB approval, the JBC team coordinated prospective recruitment to obtain fresh biospecimens. JBC research assistants contacted interested participants, scheduled study visits, performed informed consent, obtained blood samples, and delivered biospecimens of PBMCs to Dr. Chang and her team. The JBC maintained and updated the study’s recruitment database, stored study visit materials, and prepared demographic datasets for analyses and IRB purposes. Recruitment completed successfully in May 2018. In total, PBMCs were obtained from 18 homozygous controls, 16 heterozygous cases, and 4 homozygous cases. Experiments using these PBMCs are ongoing.
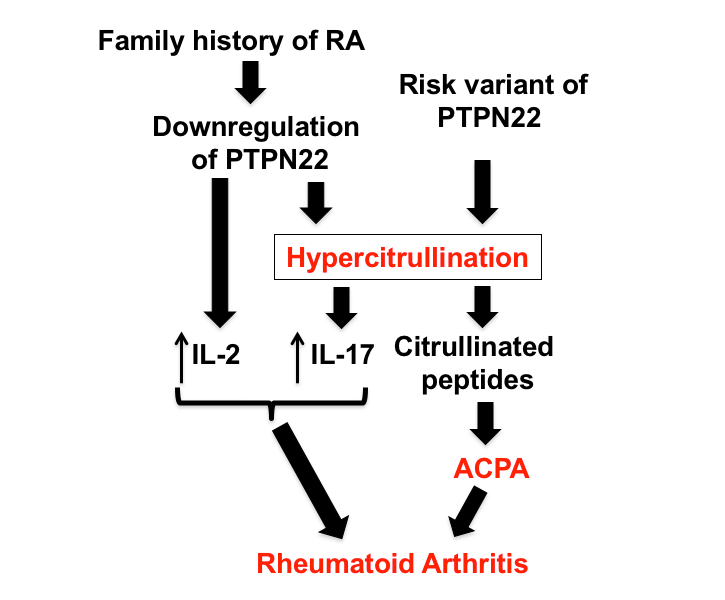
A proposed mechanism of how family history of RA and the risk variant of PTPN22 increase the risk of RA. The risk variant of PTPN22 alters its function, whereas family history of RA attenuates its expression. The altered and/or attenuated function of PTPN22 leads to enhanced production of IL-2, IL-17 and citrullinated peptides directly or indirectly through hypercitrullination. These molecular events take place before the development of ACPA and clinical symptoms of RA.
1.Chang HH, Dwivedi N, Nicholas AP, Ho IC. The W620 Polymorphism in PTPN22 Disrupts Its Interaction With Peptidylarginine Deiminase Type 4 and Enhances Citrullination and NETosis. Arthritis Rheumatol. Sep 2015;67(9):2323-2334.
2.Chang HH, Liu GY, Dwivedi N, Sun B, Okamoto Y, Kinslow JD, Deane KD, Demoruelle MK, Norris JM, Thompson PR, Sparks JA, Rao DA, Karlson EW, Hung HC, Holers VM, Ho IC. A molecular signature of preclinical rheumatoid arthritis triggered by dysregulated PTPN22. JCI insight. Oct 20 2016;1(17):e90045.